Deconvolution
An Introduction to Deconvolution
Fluorescence microscopy is a powerful technique to show specifically labelled structures within a complex environment and to provide three-dimensional information of biological structures. However, this information is blurred by the fact that, upon illumination, all fluorescently labelled structures will emit light, irrespective of whether they are in focus or not. So an image of a certain structure is always blurred by the contribution of light from structures that are out of focus. This phenomenon results in a loss of contrast especially when using objectives with a high resolving power, typically oil immersion objectives with a high numerical aperture.
However, blurring is not caused by random processes, such as light scattering, but can be well defined by the optical properties of the image formation in the microscope imaging system.
If one considers a small fluorescent light source (essentially a bright spot that is no bigger than the resolving power of the microscope), for example the following image shows how the light from a single point is actually captured as a series of expanding diffraction rings:
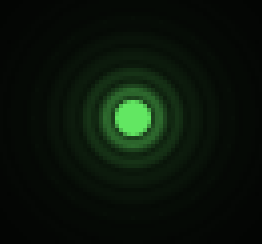
Top view (XY) of a small point of light after it has passed through a typical imaging system.
Light coming from this spot spreads out further as the spot becomes more out of focus. Under ideal conditions, this produces an "hourglass" shape of this point source in the third (axial) dimension, as shown in the image below, which is a 3D volume of the point of light.
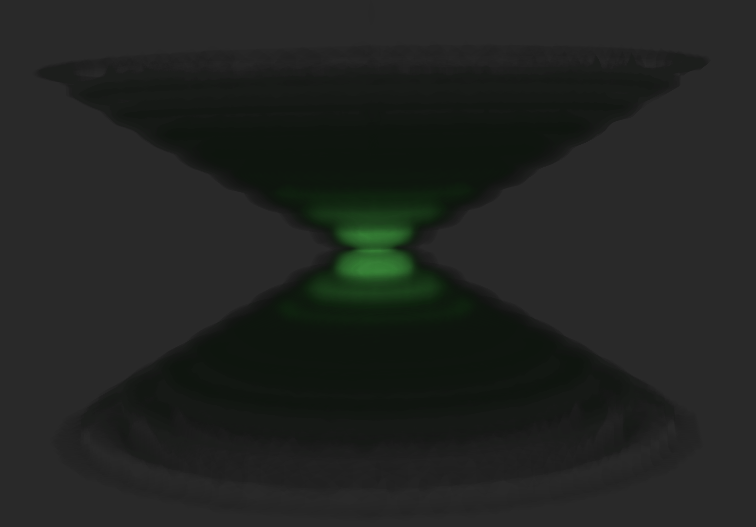
Side view (XZ) of a small point of light. Note its shape resembles that of an hourglass.
This shape is called the point spread function (PSF) of the microscope imaging system. Since any fluorescence image is made up of a large number of such small fluorescent light sources, the image is said to be "convolved by the point spread function".
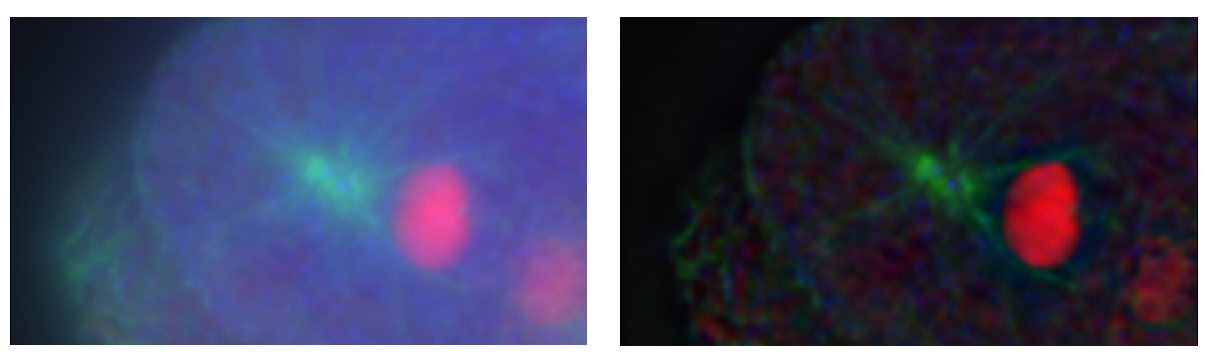
Above: Example of original image (left) and same image with deconvolution performed (right), showing a reduction of out-of focus information and improved detail.
Knowing this point spread function means that it is possible to reverse this process to a certain extent by computer-based methods commonly known as deconvolution microscopy.
There are various algorithms available for 2D or 3D deconvolution. They can be roughly classified in nonrestorative and restorative methods.
While the nonrestorative methods can improve contrast by removing out-of-focus light from focal planes, only the restorative methods can actually reassign light to its proper place of origin.
Processing fluorescent images in this manner can be an advantage over directly acquiring images without out-of-focus light, such as images from confocal microscopy, because light signals otherwise eliminated become useful information.
For 3D deconvolution, one typically provides a series of images taken from different focal planes (called a Z-stack) plus the knowledge of the PSF, which can be derived either experimentally or theoretically from knowing all contributing parameters of the microscope.
Note, that in order to optimize the output from restorative deconvolution methods, the Nyquist Sampling criterion should be followed to ensure best results.